Discover and read the best of Twitter Threads about #ESMO20
Most recents (24)
@TumorBoardTues 1/17 #TumorBoardTuesday #bcsm
CASE: 55 yo post-menopausal 👩🏽
🚫comorbidities
HR+/HER2- MBC
🦴metastases, on letrozole+palbociclib 2 yrs
🩻Scans: progression in liver
🔬Liver biopsy: ER 90% PR 90% HER2- (IHC 1+)
Tissue NGS➡️ESR1 & PIK3CA mutation
🤨What 2L tx do you recommend?
CASE: 55 yo post-menopausal 👩🏽
🚫comorbidities
HR+/HER2- MBC
🦴metastases, on letrozole+palbociclib 2 yrs
🩻Scans: progression in liver
🔬Liver biopsy: ER 90% PR 90% HER2- (IHC 1+)
Tissue NGS➡️ESR1 & PIK3CA mutation
🤨What 2L tx do you recommend?
@TumorBoardTues 2/17 #TumorBoardTuesday #BCSM #OncTwitter
🤨 What is the prevalence of PIK3CA activating mutations in metastatic HR+/HER2- breast cancer?
@Dr_RShatsky @PTarantinoMD @hoperugo @NicoleKuderer @StoverLab @CarmenCalfa @sardesai_sagar @stolaney1 @OncoAlert
🤨 What is the prevalence of PIK3CA activating mutations in metastatic HR+/HER2- breast cancer?
@Dr_RShatsky @PTarantinoMD @hoperugo @NicoleKuderer @StoverLab @CarmenCalfa @sardesai_sagar @stolaney1 @OncoAlert
@TumorBoardTues @Dr_RShatsky @PTarantinoMD @hoperugo @NicoleKuderer @StoverLab @CarmenCalfa @sardesai_sagar @stolaney1 @OncoAlert 3/17 #TumorBoardTuesday
👩🏽🏫Mini tweetorial 1👩🏻🏫
🔸⬆️PI3K/Akt/mTOR pathway➡️cell proliferation, growth & survival
🔸Class I PIK3 proteins have catalytic subunit (p110)
🔸p110 contains 4 isoforms: α, β, γ, δ
🔸p110α mut (PIK3CA encoded)➡️ resistance to ET
📚pubmed.ncbi.nlm.nih.gov/31828441/
👩🏽🏫Mini tweetorial 1👩🏻🏫
🔸⬆️PI3K/Akt/mTOR pathway➡️cell proliferation, growth & survival
🔸Class I PIK3 proteins have catalytic subunit (p110)
🔸p110 contains 4 isoforms: α, β, γ, δ
🔸p110α mut (PIK3CA encoded)➡️ resistance to ET
📚pubmed.ncbi.nlm.nih.gov/31828441/

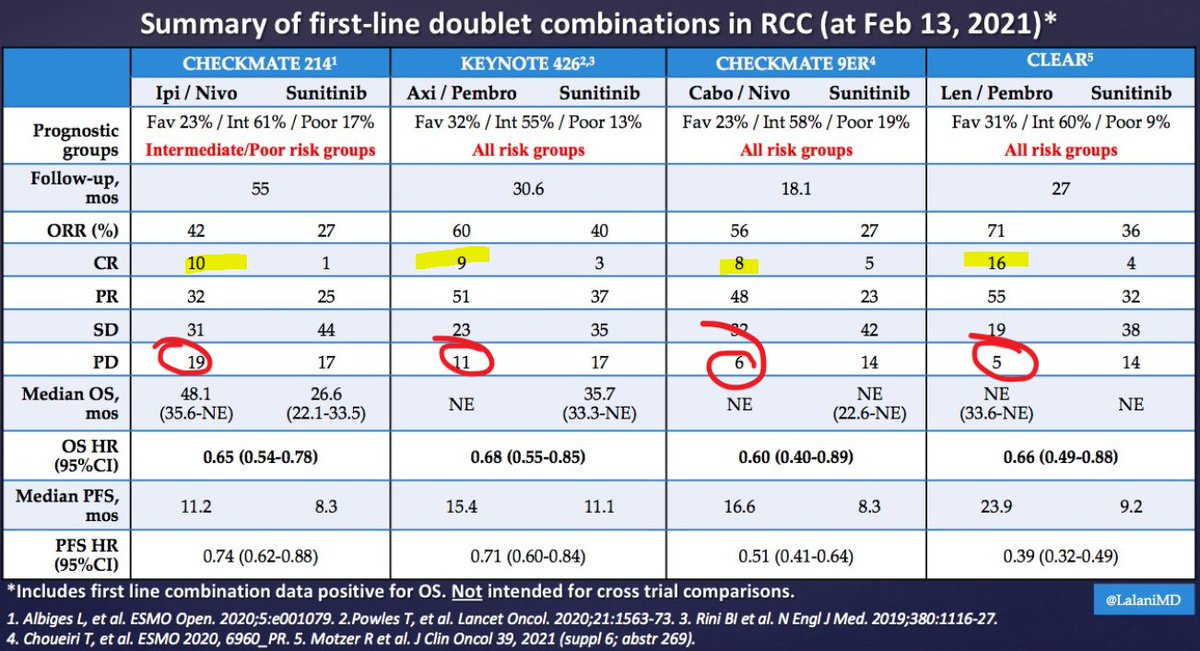
It's Monday AM post-@ASCO #GU21 & clinic starts in a couple of hours! Lots to process - I'll try to tackle optimal 1L tx for #kidneycancer. I'll make a case for cabo/nivo, leaning on the beautiful (& timely) tables below from @lalaniMD, @SoaresAndrey & @brian_rini (1/15) 


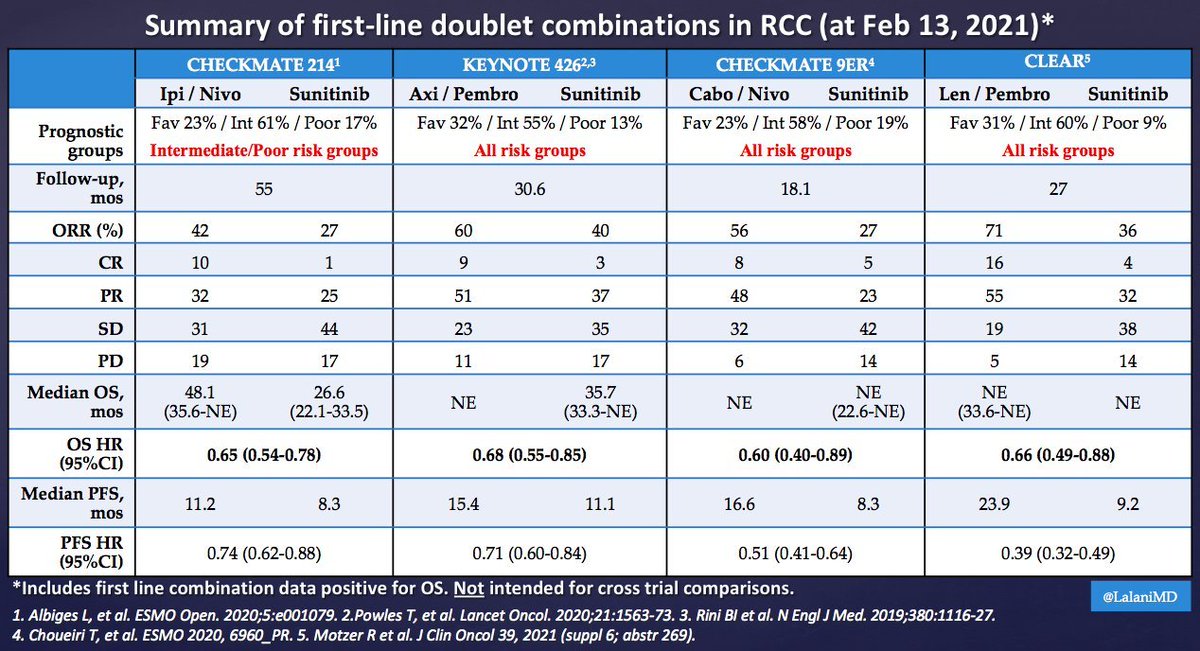

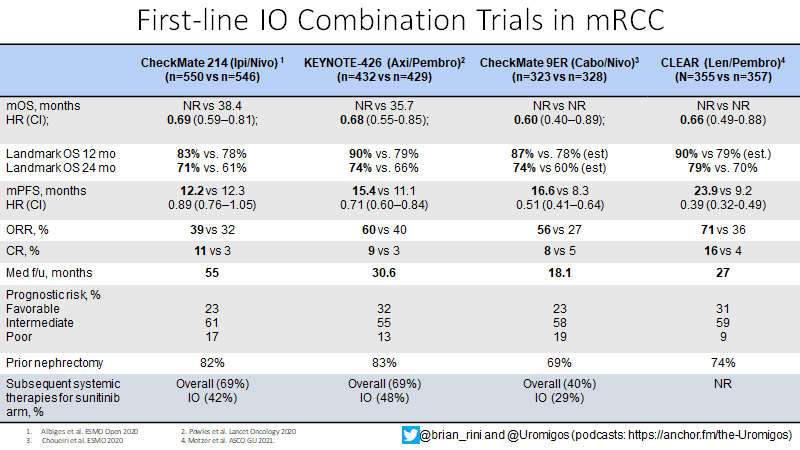
What about IO/IO? We have long f/u w #CM214 data w nivo/ipi, no doubt (@AlbigesL et al in @myESMO Open). And treatment-free interval discussed by McDermott @BIDMChealth is no doubt impt. But we've known data not as impressive for favorable risk (2/15) 



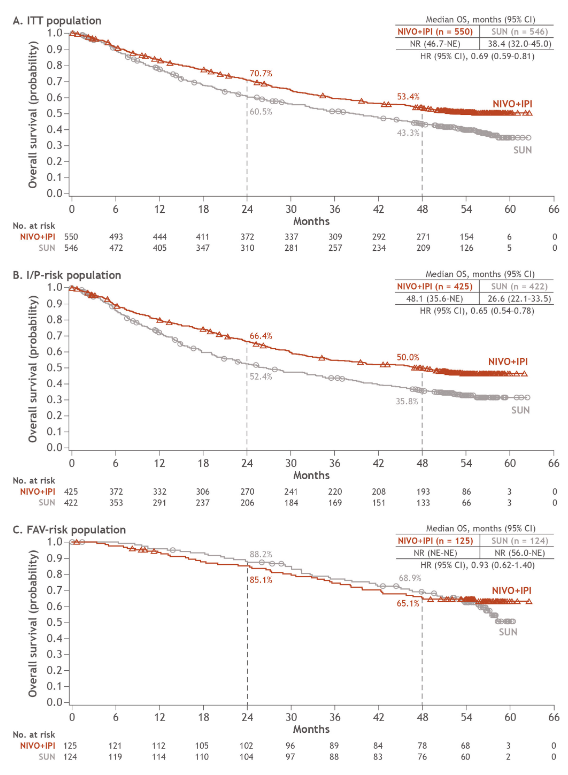

And furthermore, as @ERPlimackMD points out in another tweet, impt to look at primary PD rates (seen in @lalaniMD's table) - nivo/ipi at 19%!!! CR rate used to be something we highlighted w nivo/ipi, but now comparable across studies (3/15) 
Folks! We return for #HOTrainees with the exciting #Day2 @myESMO #ESMO20 and some more #practice relevant studies in #breastcancer #ProstateCancer #lungcancer #GI, so sit back, relax and lets go through some data (#HO #trainee-style!) Shout out to @peters_solange @OncoAlert
1. #BreastCancer: We have #monarchE and #IMPassion031 hoping to hear from experts @ErikaHamilton9 @NicoleKuderer @DrSGraff @matteolambe @tmprowell @GeorgeSledge51 @VukovicPetra for more insights- please link to your discussions here for #trainees:
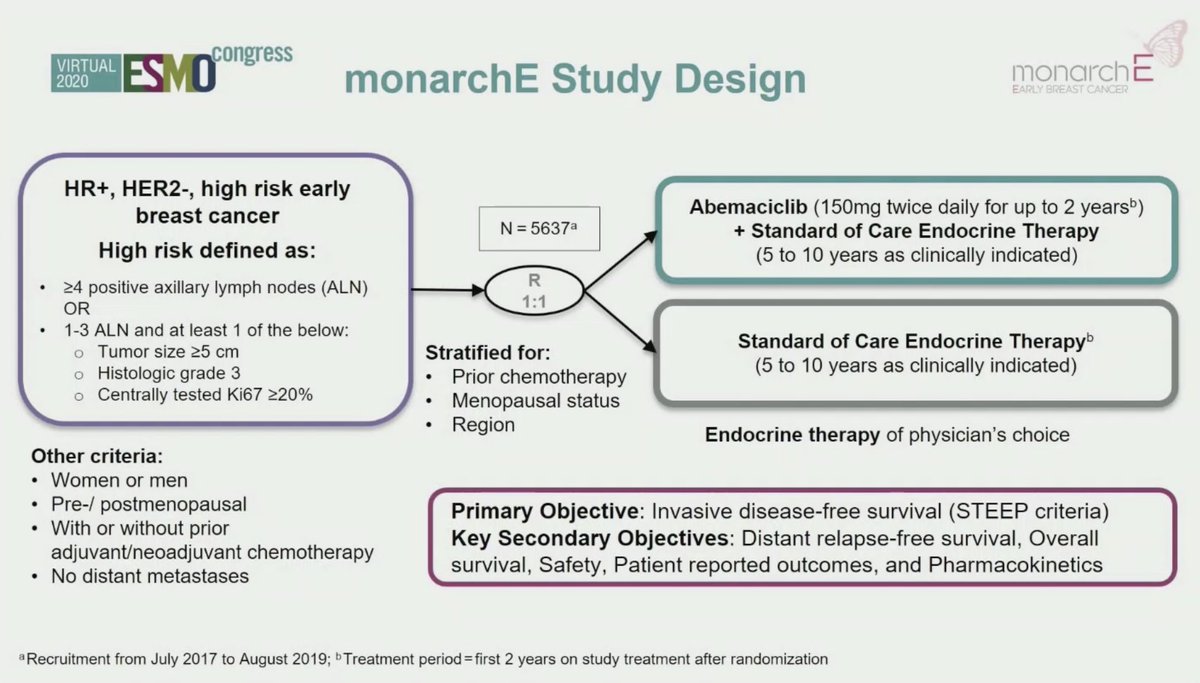
1. A) #monarchE: use of #abemiciclib in HR+, HER2-,high risk #EBC in addition to endocrine therapy.
Current #SOC: adjuvant ET (5-10 years)
#monarchE: #Abemaciclib + ET iDFS HR 0.747, here's a great summary by @ErikaHamilton9 for @OncoAlert :

Current #SOC: adjuvant ET (5-10 years)
#monarchE: #Abemaciclib + ET iDFS HR 0.747, here's a great summary by @ErikaHamilton9 for @OncoAlert :

#ESMO20 Practice changing data presented by Dr. Cecile Le Pechoux @GustaveRoussy. The prospective, randomized phase III LUNG-ART study explores the benefit of post-operative radiotherapy for completely resected #NSCLC: no OS benefit seen. #LCSM @OncoAlert 

#ESMO20 Post-operative radiotherapy found to be detrimental after resection for pN0 and pN1 NSCLC but unclear role in pN2. Data supporting its benefit but lack of prospective studies. Had been standard for many in pN2 (including myself). #LCSM @OncoAlert 



#ESMO20 The LUNG ART phase III trial (IFCT-0503) enrolled patients with completely resected N2 NSCLC and randomized 1:1 to 3D conformal post operative radiotherapy (54 Gy over 5.5 weeks). Median follow up nearly 5 years. #LCSM @OncoAlert 



#ESMO20 Dr. @HelenaYu923 presents data on patritumab deruxtecan (U3-1402), a HER3-ADC for patients with #EGFR mutant NSCLC. #LCSM @OncoAlert 

#ESMO20 Update on poziotinib in #HER2 mutant #NSCLC by Mark Socinski from the ongoing phase II ZENITH20 study. #LCSM @OncoAlert @AdventHealth 

#ESMO20 There are 7 cohorts to ZENITH20, 3 have completed accrual. This presentation focuses on cohort 2, #HER2 mutant NSCLC. Heavily pretreated population with most having received IO therapy and some with prior HER2 therapy as well. #LCSM @OncoAlert 


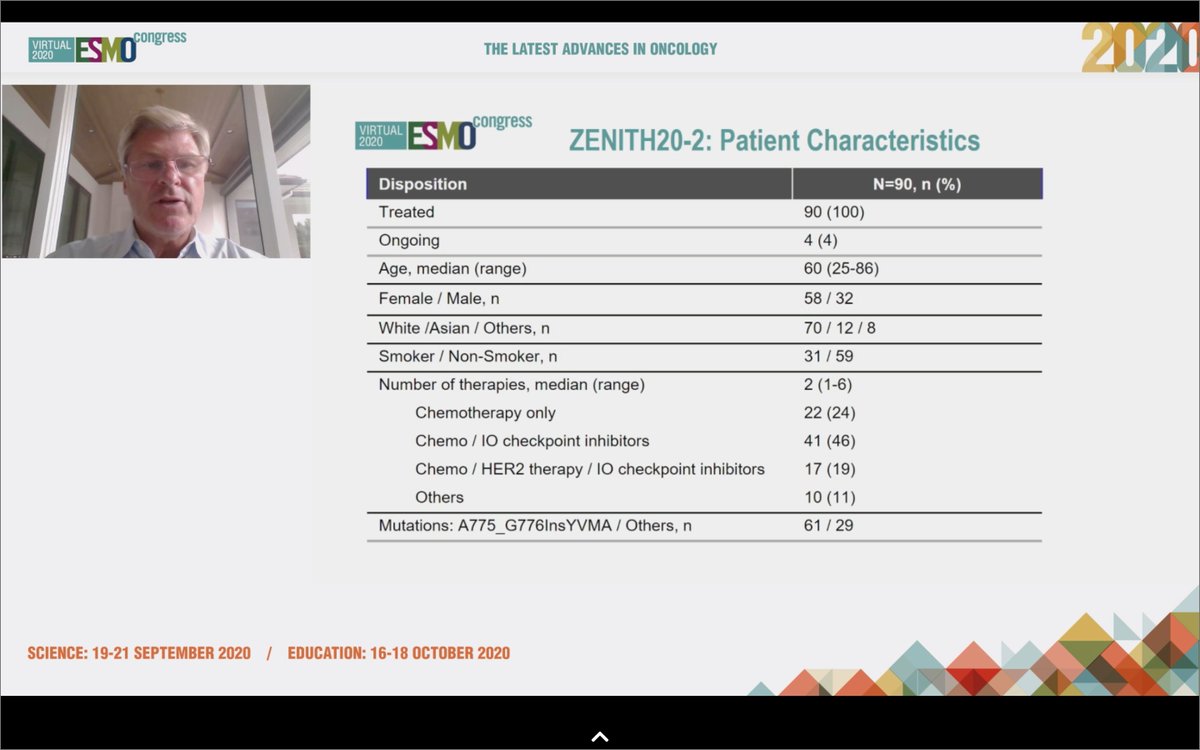
#ESMO20 Poziotinib in #HER2 mutant NSCLC had a response rate of 27.8%. Duration of response 5.1 months, median time on treatment 3.7 months, median PFS 5.5 months. #LCSM @OncoAlert 

#ESMO20 Wonderful discussion of the VEGF/EGFR oral presentations given by @LeciaSequist framing the results in the current landscape. #LCSM @OncoAlert 

#ESMO20 Targeting angiogenesis in NSCLC is an effective strategy, with several regimens approved including in patients with #EGFR mutant NSCLC, reflected in @NCCN guidelines though conspicuously absent is gefitinib + chemotherapy, which offered an OS benefit over TKI alone. #LCSM 





#ESMO20 There's a history between EGFR and VEGF. The pattern: improved PFS but not OS. Do we need OS? Definitely if QoL is impacted. 



#ESMO20 Randomized phase II study of osimertinib alone or with bevacizumab in #EGFR T790M NSCLC by Prof Yukihiro Toi (West Japan Oncology Group 8715L). #LCSM @OncoAlert 

#ESMO20 After a 6 patient lead-in, patients randomized 1:1 to osimertinib 80mg alone or with bevacizumab 15mg/kg q3w. Primary endpoint here was PFS. #LCSM @OncoAlert 

#ESMO20 81 patients randomized, 22-30% with brain metastases. Slight imbalance in del19 vs L858R. Given the known biologic differences between these mutations, I do think we need to stratify for specific mutation in randomized studies going forward. #LCSM @OncoAlert 



#ESMO20 Results from the randomized, double blind ACTIVE trial (CTONG 1706) of apatinib + gefitinib (vs placebo + gefitinib) as 1L treatment for #EGFR mutant NSCLC. Apatinib is a VEGFR2 TKI. Inevitable parallels to RELAY (erlotinib + ramucirumab). #LCSM @OncoAlert 



#ESMO20 Treatment naive EGFR+ mNSCLC randomized to oral apatinib 500mg qday or placebo, both with gefitinib 250mg qday. Stratified by mutation, sex, PS. Primary endpoint PFS. #LCSM @OncoAlert 

#ESMO20 The ACTIVE trial randomized 313 patients with a median follow up of 15.8 months. Brain metastases present in 32.5% of combination group and 26.3% of gefitinib monotherapy (unclear to me if treated or not). #LCSM @OncoAlert 





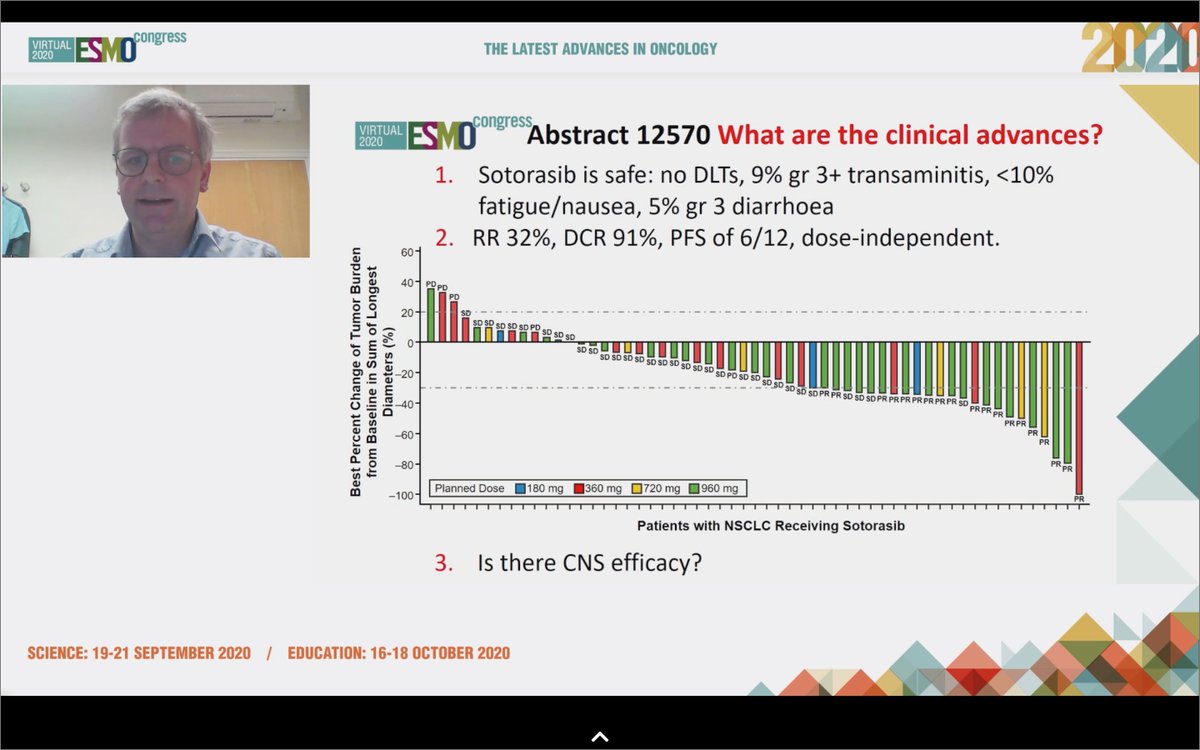
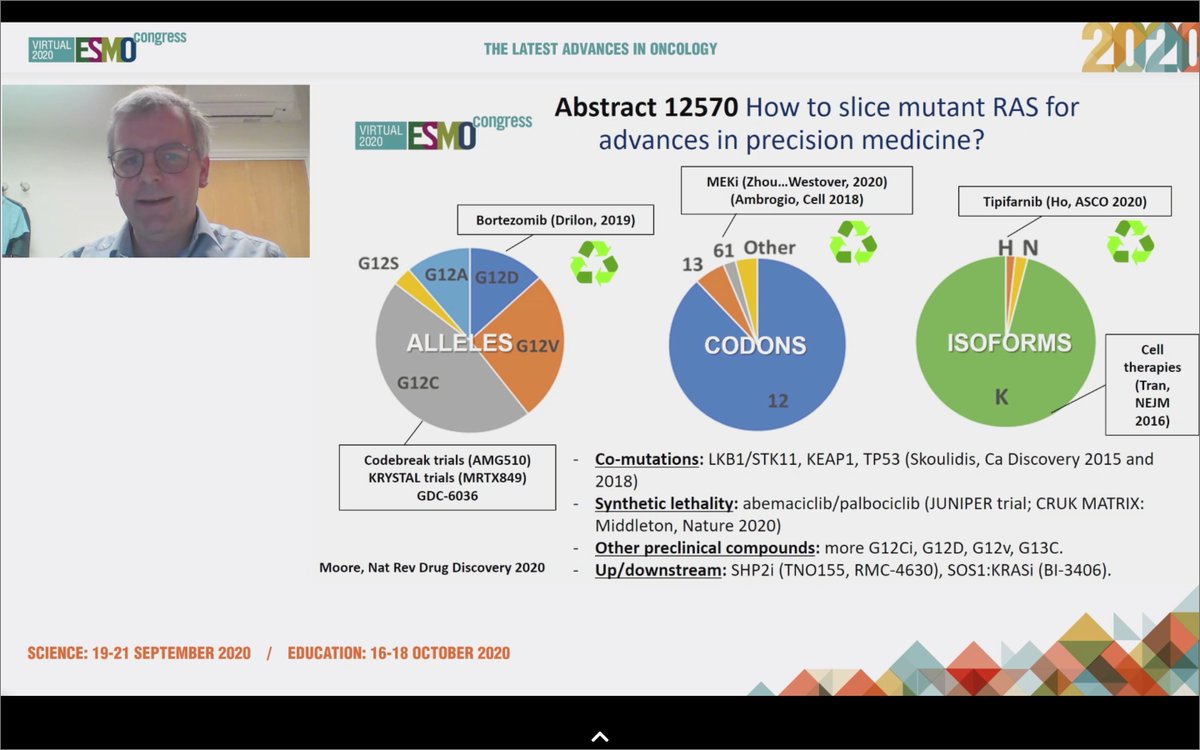
#ESMO20 Well done discussion by Dr. Colin Lindsay who wins for most random @Twitter handle - @mylovelypinata. Helpful reminder of the journey leading to direct #KRAS inhibitors and the achievements of medicinal chemistry leading to agents like AMG 510 and MRTX849. #LCSM 





#ESMO20 Sotorasib was safe and effective in #KRAS G12C NSCLC. Safety profile will allow for combinations: what are the right partners? Can we combine with IO? Do we need a phase III vs docetaxel? @mylovelypinata says absolutely, we do. And other KRAS mutations being targeted too! 



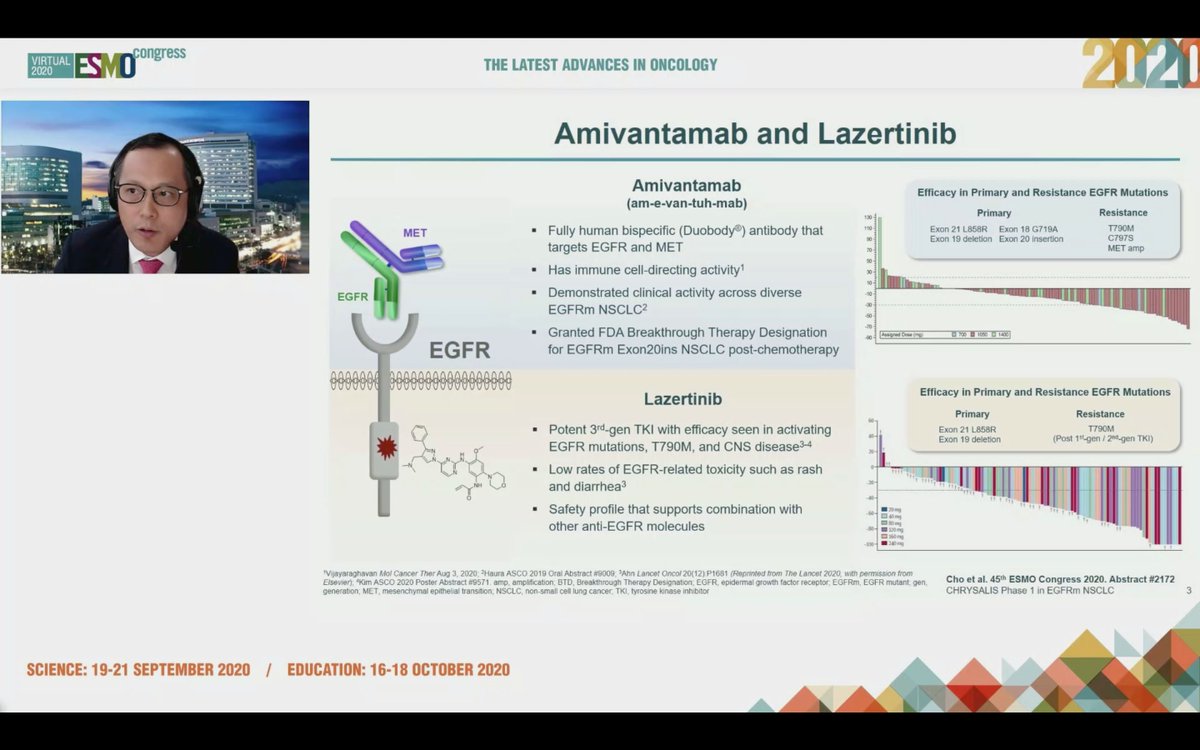
#ESMO20 Commenting on CHRYSALIS - amivantamab and lazertinib was safe and effective in osi-resistant and naive populations. Too early for cross trial comparisons but it seems as though IV drugs for #EGFR NSCLC are back. #LCSM #OncoAlert 


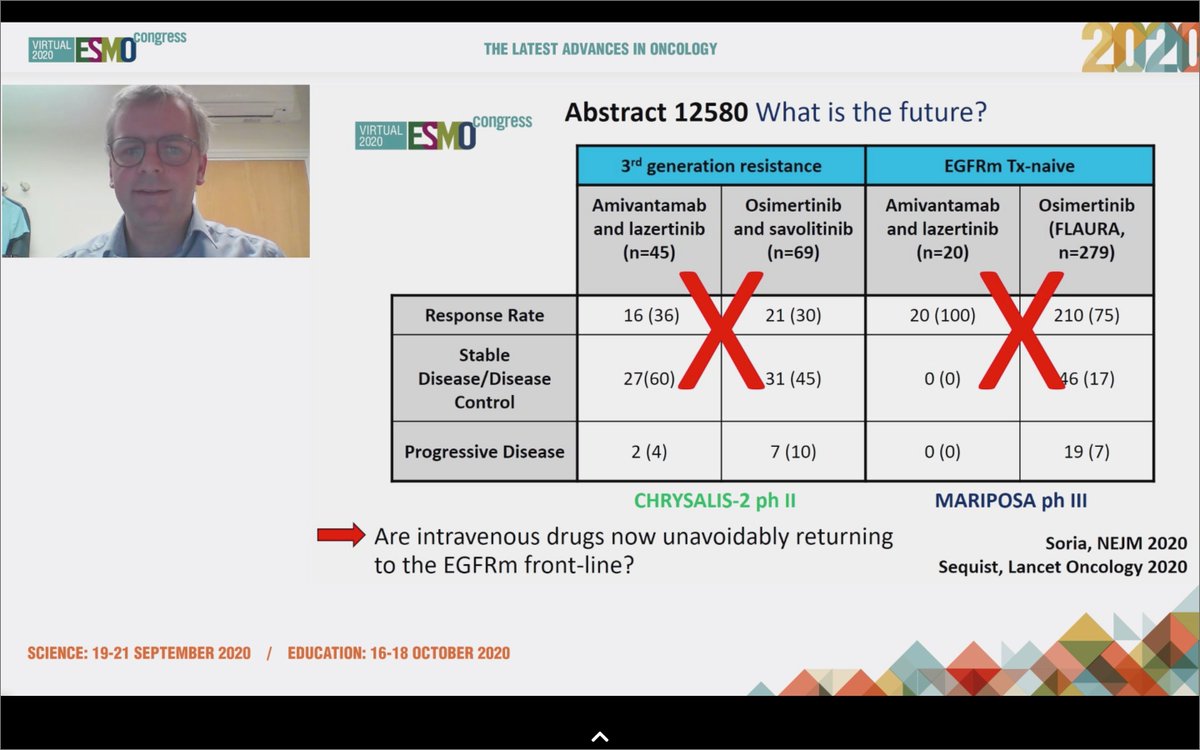
#ESMO20 Byoung Cho with important results from CHRYSALIS: amivantamab (JNJ-611863372, EGFR-MET bispecific) with lazertinib (3rd gen #EGFR TKI) in patients with sensitizing EGFR mt #NSCLC. Strong work from team including @Jbauml @AlexSpiraMDPhD @JSabari @RachelSanbornMD #LCSM 

#ESMO20 Much anticipated results from CodeBreaK100: AMG 510 (sotorasib) in #KRAS G12C #NSCLC by @DavidHongMD building on exciting data seen over the past two years for this huge unmet need. #LCSM @OncoAlert @myESMO 

#ESMO20 KRAS G12C occurs in 13% of NSCLC, 3-5% CRC, and other tumors as well. Up until recently, considered "undruggable". Sotorasib is a highly selective KRAS G12C inhibitor that traps KRAS in its inactive GDP bound state. #LCSM @OncoAlert 

#ESMO20 CodeBreak100 is a phase I escalation/expansion trial of sotorasib monotherapy in KRAS G12C tumors. Primary endpoint was safety. Escalation established preferred dose of 960mg qday. #LCSM @OncoAlert 
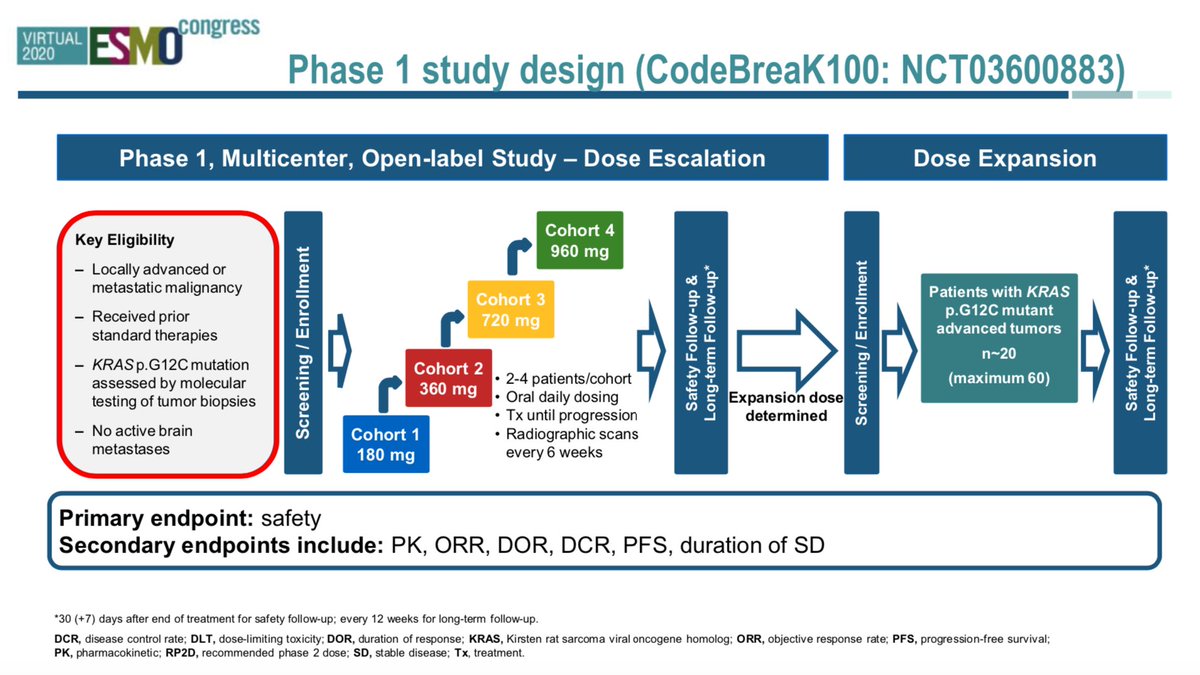
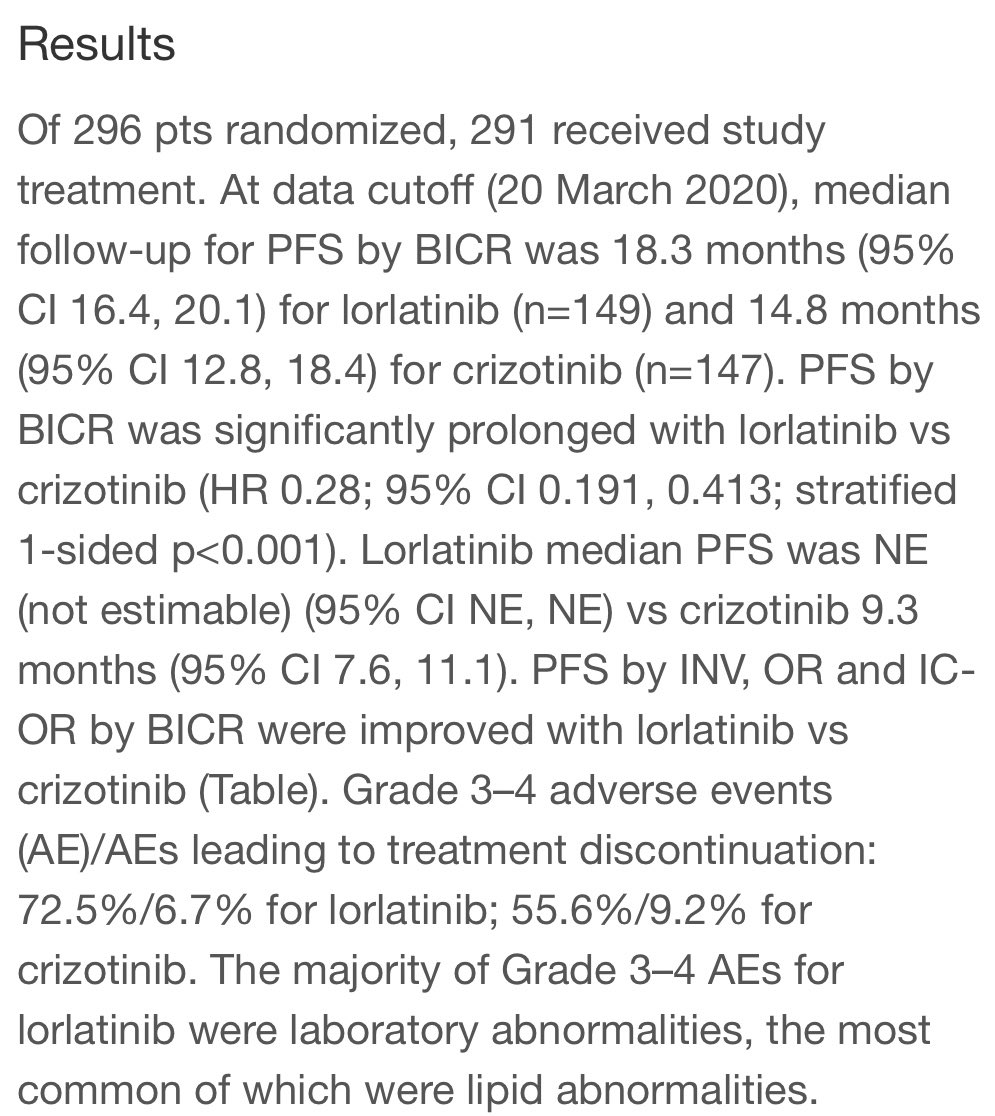
I am going through the CROWN trial presentation, expertly presented at #ESMO20 by @bensolomon1. Also kudos to @EnriquetaFelip @JulienMazieres @TonyMok9 and the rest of the authors (those were the ones I could find on twitter). This is an important study, and analysis is complex 

Here, of course, is the slide everyone is talking about. HR 0.28 for PFS favoring lorlatinib. This is undoubtedly an impressive HR, but how do these data compare to prior series and studies? #LCSM 

@myESMO #ESMO20 as a #trainee can be #overwhelming! So many good studies, some more #practicechanging then others, if you missed some and want to understand (albeit at a simplistic #trainee level), sit back, relax and enjoy as we go through some great data #ESMO20 @OncoAlert
1. #NSCLC: 2 major studies #ADAURA #CROWN for adjuvant #EGFRmNSCLC, and advanced #ALK+ experts can provide better perspective @JackWestMD @n8pennell @StephenVLiu @AMansfieldMD @CharuAggarwalMD @NarjustDumaMD @GlopesMd @DevikaDasMD @OncoAlert
1. A) #ADAURA: Stage IB-IIIA #resected #NSCLC with #EGFRm treated with #Osimertinib vs #placebo [SOC prior to this was adjuvant chemotherapy [cisplatin-based doublet based on #LACE metanalysis- pubmed.ncbi.nlm.nih.gov/18506026/] showed improvement in #DFS @NEJM nejm.org/doi/full/10.10…
#ESMO20 Discussion of CROWN led by @christine_lovly. Markedly positive results from the study. Limitations aside, lorlatinib compares very favorably to other 2G TKIs - alectinib, brigatinib, ensartinib. Toxicity is different. 




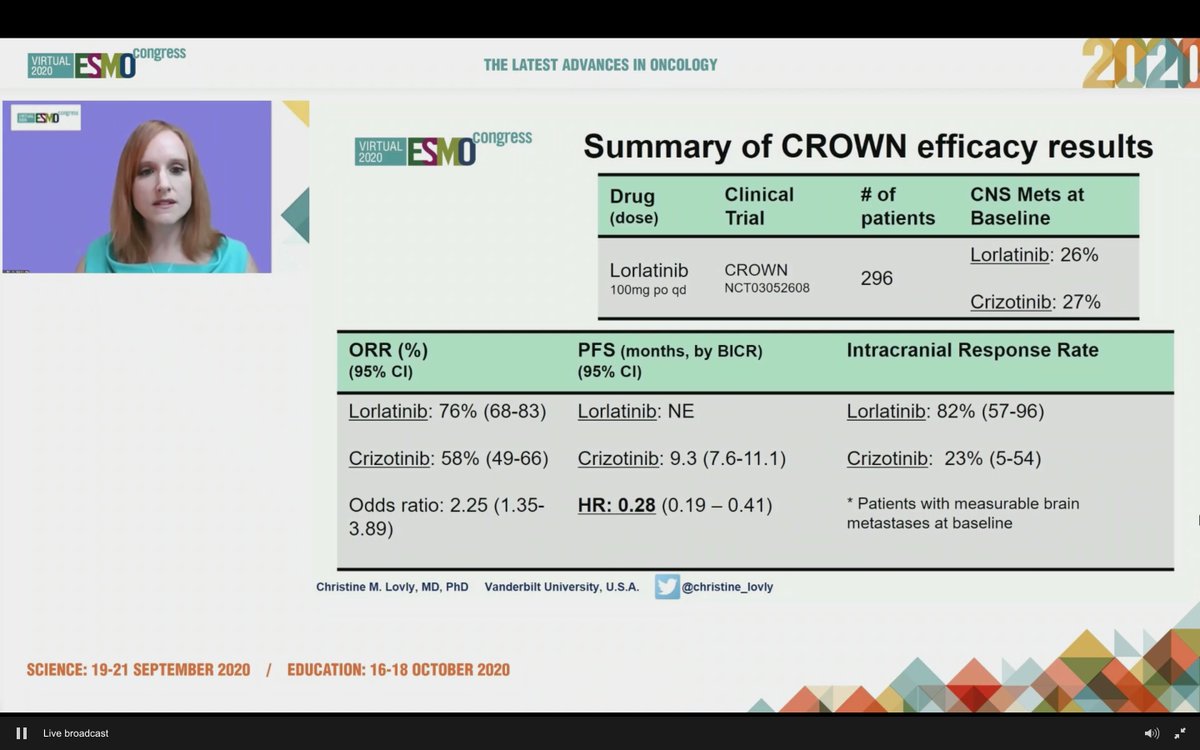

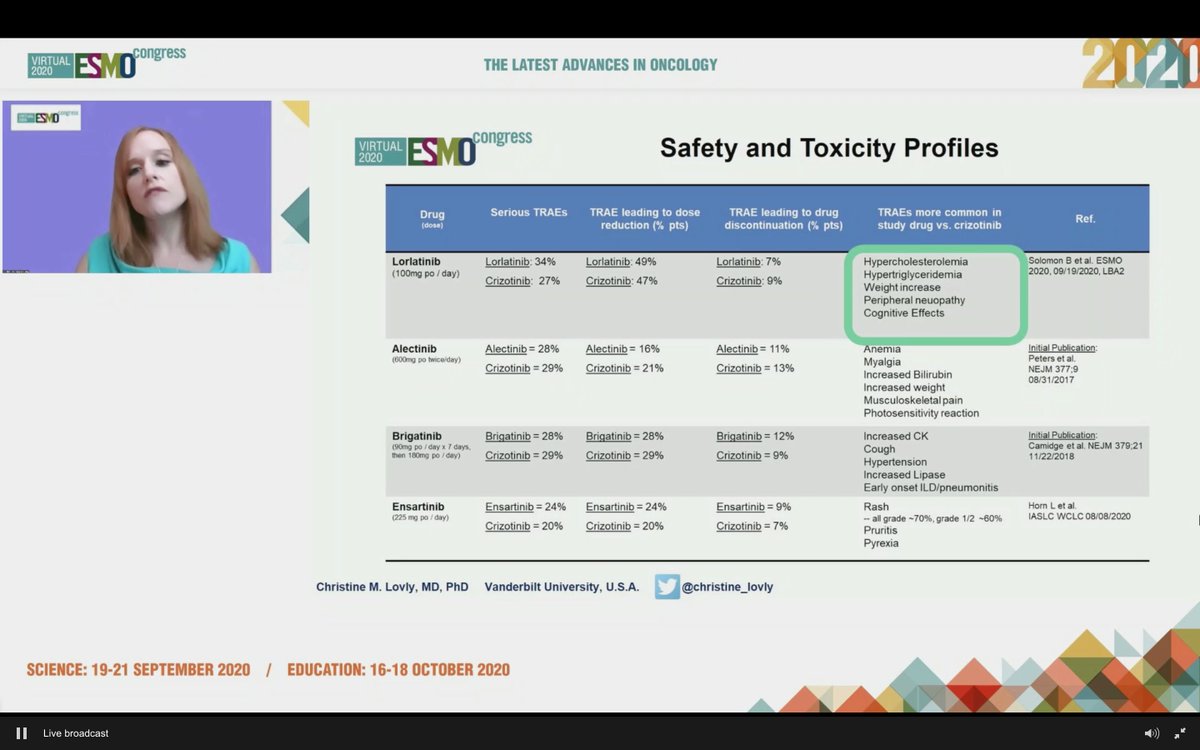
#ESMO20 We will need to learn more about resistance to 1L lorlatinib and seek biomarkers to help guide initial therapy in #ALK NSCLC but having more options is a good thing and overall, the HR and CNS efficacy are very compelling. #LCSM @OncoAlert 



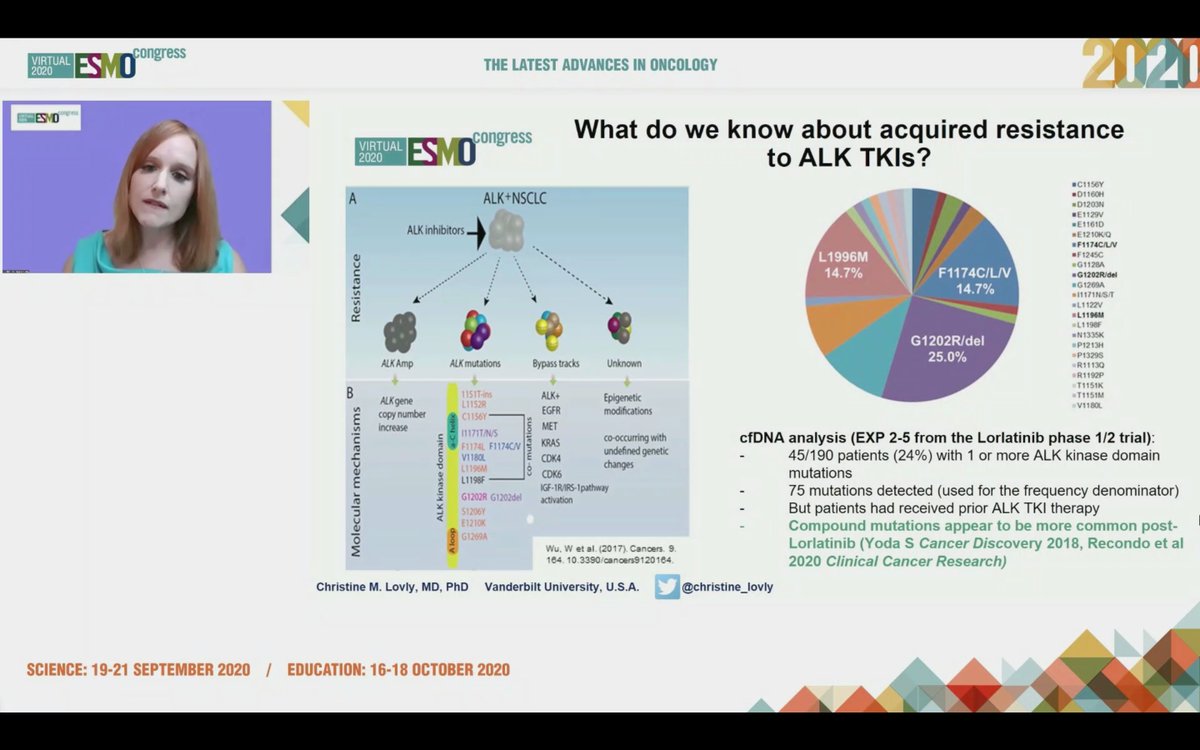

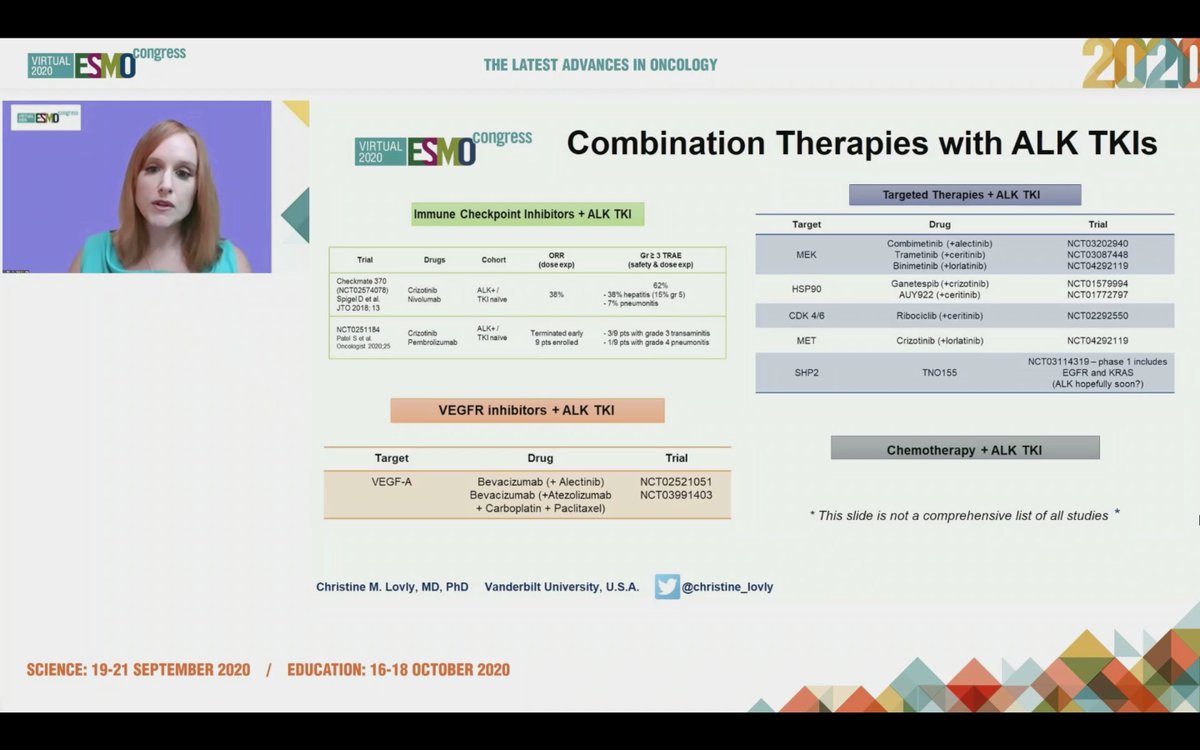
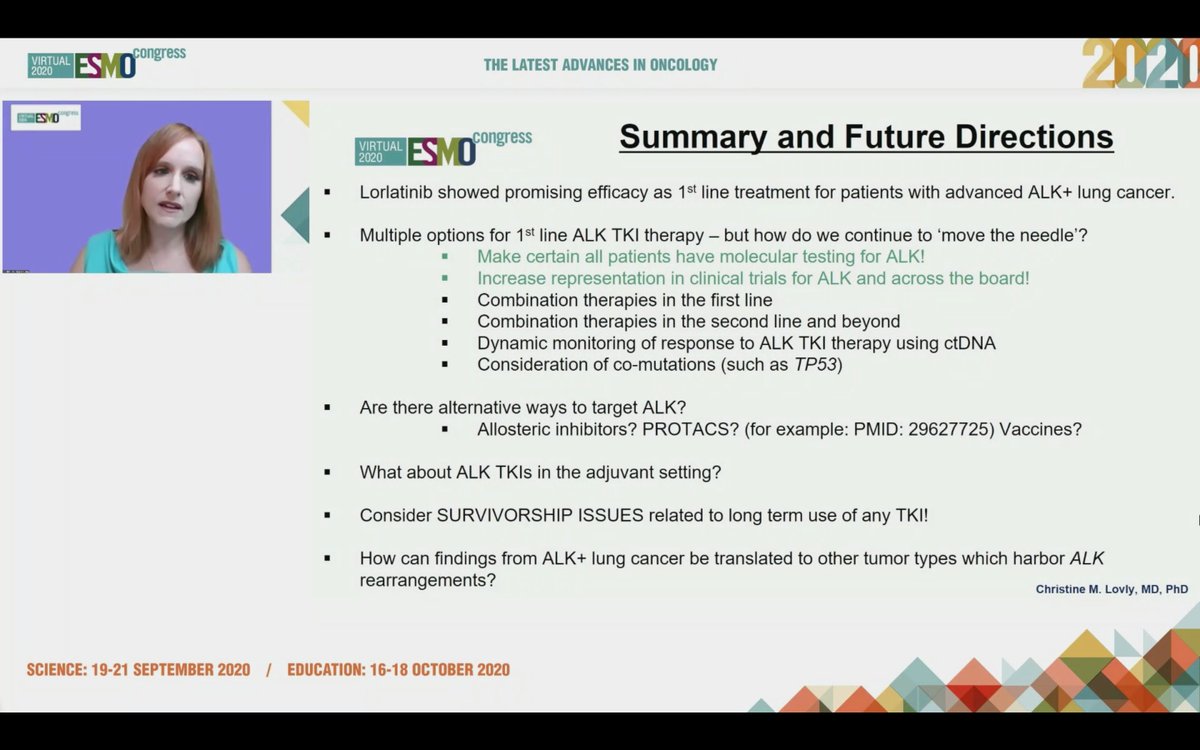
#ESMO20 Tremendous progress seen in #ALK NSCLC and @christine_lovly makes a note to thank the important work done by our patient advocate colleagues @ALKLungCancer @alk_fusion #LCSM 
#ESMO20 Discussion of the phase III CROWN trial results (planned interim analysis): lorlatinib vs crizotinib in treatment naive #ALK #NSCLC by @bensolomon1 - potentially practice changing data in an important subset of lung cancer. #LCSM @OncoAlert 

#ESMO20 #ALK fusion positive NSCLC is an important subset where we have very potent and active agents with PFS measured in years - but always room for improvement. #LCSM @OncoAlert 

#ESMO20 Study design for CROWN shown here. Standard 1:1 randomization to lorlatinib 100mg qday vs crizotinib 250mg bid, stratified by Asian vs not and presence or absence of brain metastases. Primary endpoint was PFS by BICR. #LCSM @OncoAlert 





#ESMO20 Key update on #ADAURA regarding CNS disease recurrence with adjuvant osimertinib vs placebo after resected stage IB-III #EGFR mutant NSCLC. #LCSM @OncoAlert @myESMO 

#ESMO20 We saw at #ASCO20 the positive results from #ADAURA where adjuvant osimertinib significantly improved DFS in resected stage II/III NSCLC (DFS HR 0.17). Pre-specified analysis CNS recurrence given tropism of the disease and CNS efficacy of osimertinib. #LCSM 







#ESMO20 Patients receiving osimertinib were less likely to recur overall but when there was recurrence, it was less likely to be distant. #LCSM @OncoAlert 

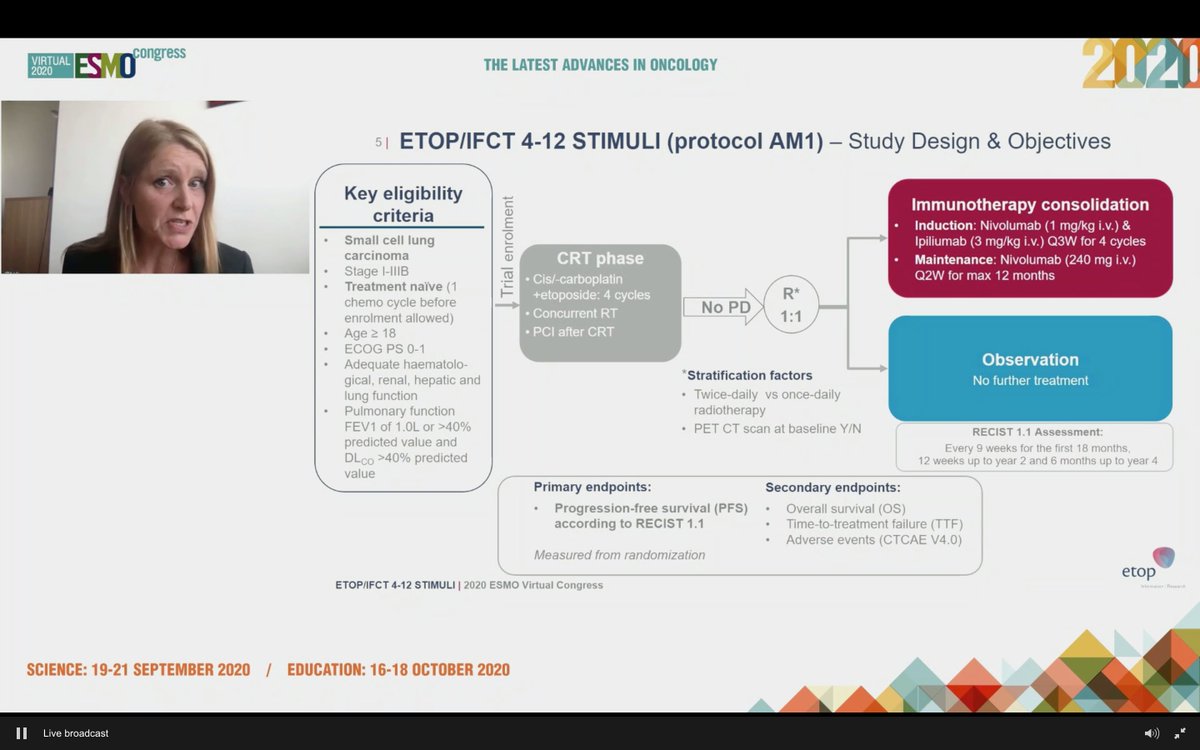
#ESMO20 Eagerly awaited presentation by @peters_solange on consolidation nivolumab and ipilimumab (vs observation) for patients with LS-SCLC after chemoradiation: ETOP/IFCT 4-12 STIMULI trial #LCSM @OncoAlert @myESMO 

#ESMO20 While we have adopted IO for ES-SCLC, its role in LS-SCLC is not yet known, though outcomes in LS-SCLC are still poor. Nivo/ipi has activity in SCLC, but note that the consolidation/maintenance approach in ES-SCLC was not successful. #LCSM 



#ESMO20 Study design outlined here: enrolled at diagnosis or after 1 dose of chemotherapy to allow referrals (smart design). Standard concurrent CRT followed by 1:1 IO vs observation. Used nivo 1mg/kg and ipi 3mg/kg q3w x 4 cycles with maintenance nivolumab (not low dose ipi). 
#ESMO20 Presentation of the phase II PRINCEPS trial of neoadjuvant atezolizumab monotherapy in resectable NSCLC by @BenjaminBesseMD from @GustaveRoussy #LCSM @OncoAlert @myESMO 
#ESMO20 Building on other studies showing 17-45% rates of major pathologic responses (MPR) but focused on the translational opportunities with these neoadjuvant trials #LCSM @OncoAlert 

#ESMO20 Report of the IFCT-1601 IoNESCO study of neoadjuvant durvalumab monotherapy for resectable #NSCLC presented by @MarieWislez in the Proffered Paper session chaired by @LudaBazhenovaMD @FlorianaMorgill #LCSM @OncoAlert 

#ESMO20 Neoadjuvant checkpoint inhibitor monotherapy has shown efficacy in previous studies of resectable stage I-III NSCLC with major pathologic responses (MPR) seen in 17-45% of patients. @OncoAlert #LCSM 

#ESMO20 Study design of IoNESCO IFCT-1601: included stage IB, II, IIIA (non-N2), treatment was three doses of durvalumab every 2 weeks followed by surgery within 2-14d of resection. Primary endpoint was feasibility of resection with 90d mortality as a secondary. #LCSM @OncoAlert 



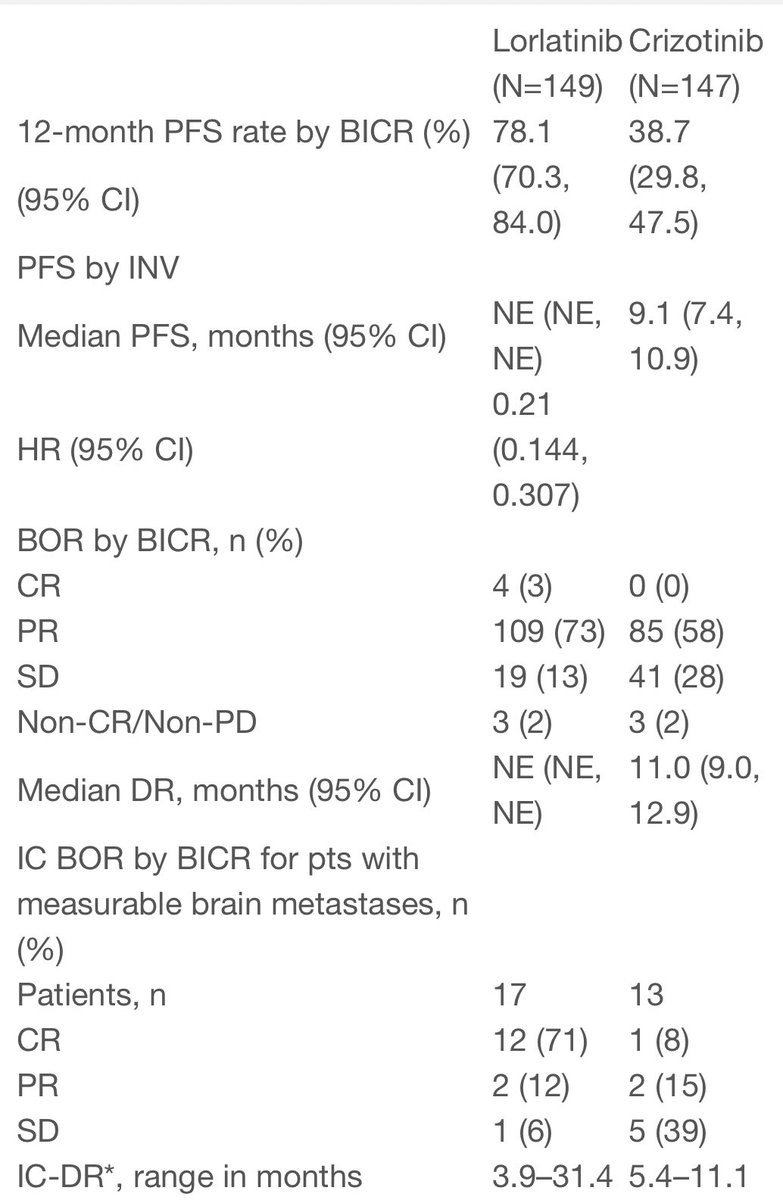
#ESMO20 Presidential abstracts now online @myESMO including CROWN results. Phase 3 trial of lorlatinib vs crizotinib. We learned by press release the the study was positive for PFS. The question was HOW positive... #LCSM @OncoAlert 



#ESMO20 Answer: VERY positive. PFS HR: 0.21!! Median PFS with crizotinib was 9.1, RR 58%. Median with lorlatinib not reached, RR 76%. Treatment dc due to AE lower with lorlatinib (7% vs 9%). Eager to see the full presentation by @bensolomon1 #LCSM 

I get the feeling we’re not done with FDA approvals for NSCLC this year...
#ESMO20 With some of the technical limitations, grateful for this discussion of the mesothelioma #MPM oral abstracts by @GautschiOliver @OncoAlert 

#ESMO20 @GautschiOliver reviews the history of immunotherapy in #MPM, with early single arm trials showing efficacy but disappointing randomized trials of tremelimumab and of pembro vs chemo. Still, responses were seen, including his own patient with a 3y response. @OncoAlert 




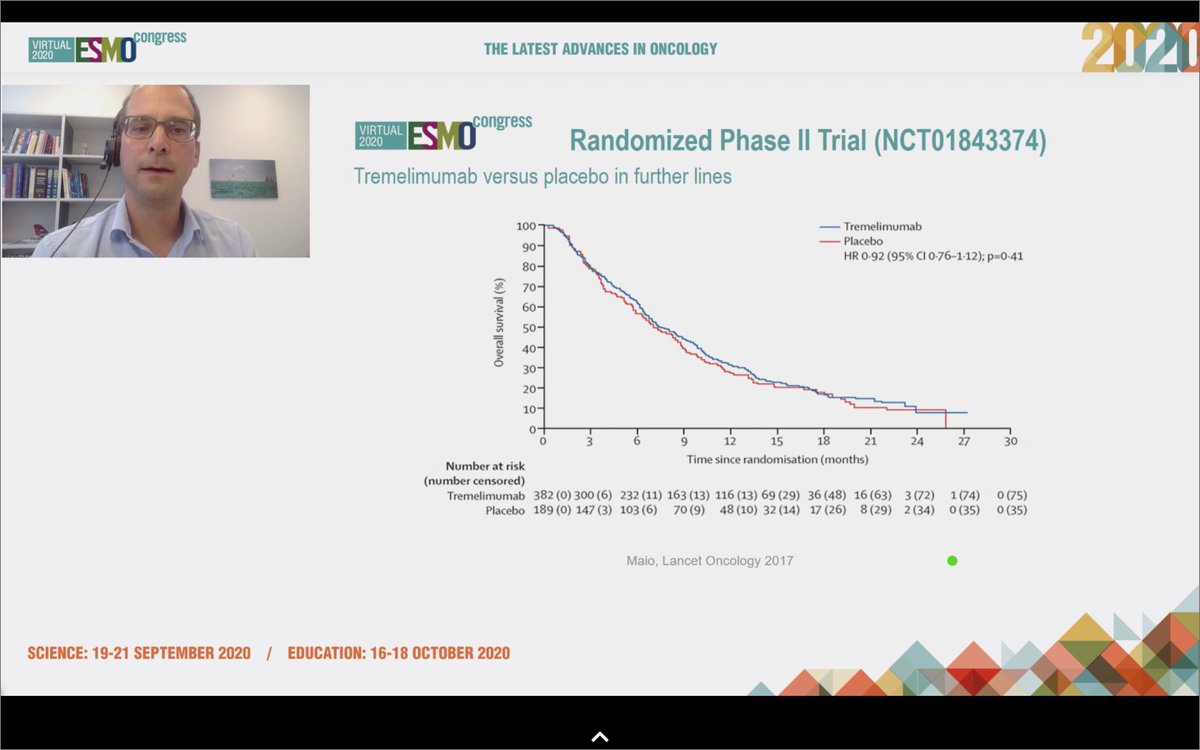
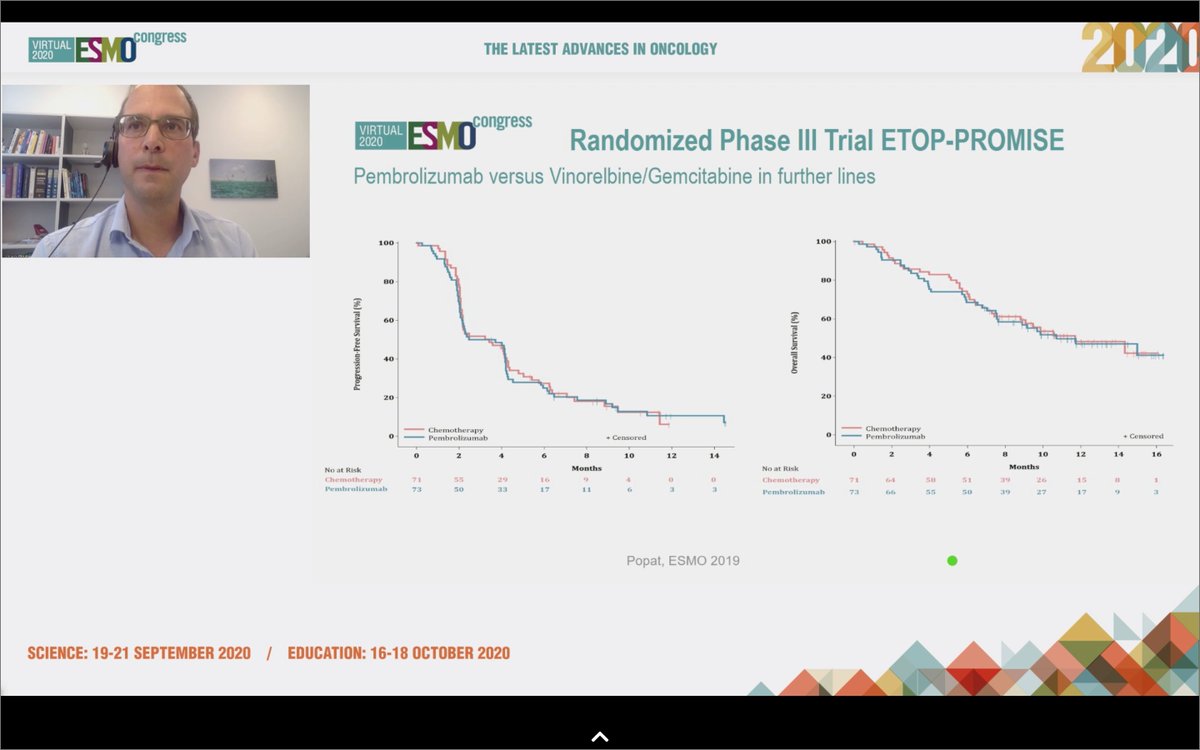

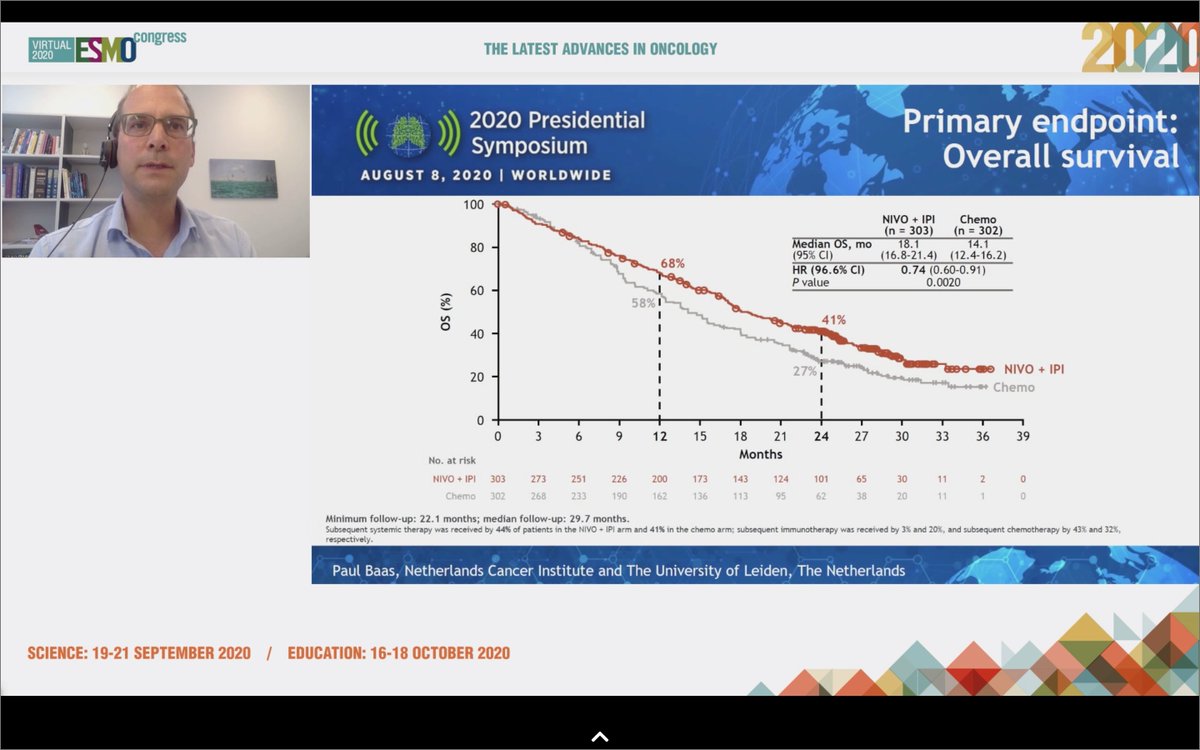
#ESMO20 That finally changed with #WCLC20 @IASLC with CheckMate 743 when first-line nivo/ipi improved OS compared to chemotherapy (OS HR 0.74), particularly in the non-epithelioid subtype (OS HR 0.46). @OncoAlert 



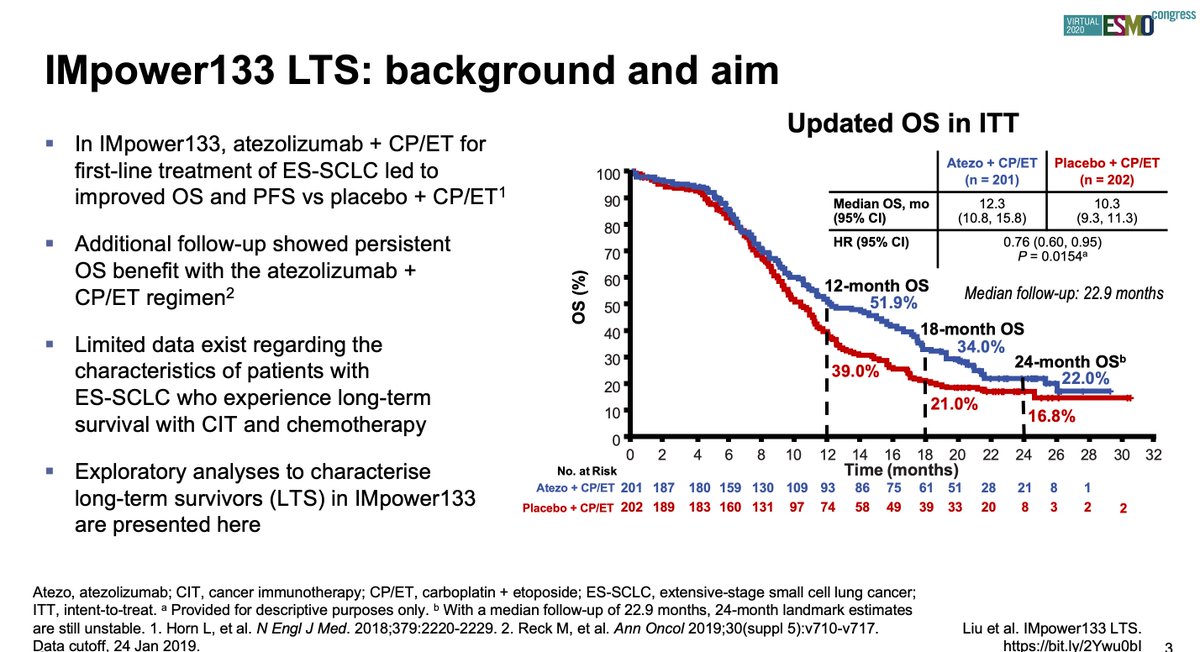
#ESMO20 Grateful for the chance to present characterization of long-term #SCLC survivors with atezolizumab and carboplatin/etoposide in IMpower 133. #LCSM @IASLC @OncoAlert 

#ESMO20 IMpower 133 was a placebo controlled, double blind, randomized phase III trial for patients with treatment naive ES-SCLC that showed the addition of atezolizumab to 1L carboplatin + etoposide improved PFS and OS, leading to FDA and EMA approval in 2019. #LCSM @OncoAlert 
#ESMO20 Happy to share the first results of RAIN-701, a phase II study of #tarloxotinib in patients with NSCLC harboring an #EGFR exon 20 insertion or an activating #HER2 mutation and any solid tumor with an #NRG1 or ERBB gene fusion. #LCSM @OncoAlert 

#ESMO20 Tarlox is given as an inactive prodrug that embeds in the cell membrane. Only when it encounters hypoxia does it undergo single electron reduction to fragment to a cell permeable, pan-HER, irreversible TKI with subnanomolar potency at EGFR, HER2 and HER4. @OncoAlert #LCSM